
Cardiac Science Powerheart G5
- This AED package includes:
- Cardiac Science Powerheart G5 fully-automatic AED
- Cardiac Science Powerheart G5 Intellisense battery
- Cardiac Science Powerheart G5 elektrodes for Adults
- Software
- USB Cable
- Cardiac Science Powerheart G5 Manual
Cardiac Science Powerheart G5 fully-automatic AED
AED with CPR Sensor and more Advanced Technologies
The Cardiac Science Powerheart G5 fully-automatic makes the difference in life-saving resuscitation. This defibrillator guides you with voice instructions, how quickly the chest compression should be performed.
The Powerheart G5 AED can be equipped with a CPR sensor. With this advanced technology direct feedback is given during CPR about the speed and depth of chest compressions. These two factors largely determine the chances of survival of the victim with a circulatory arrest. The Cardiac Science G5 AED supports the rescuer through voice commands.
The reliability of the Cardiac Science Powerheart G5 fully-automatic is enormous. This defibrillator is produced under the supervision of the FDA. Therefore the quality standard is the highest attainable. FDA stands for Food & Drugs Authority. The FDA monitors the production of medical equipment in USA and is also the body that undergoes the strictest regulations worldwide for manufacturers of medical equipment.
AEDs are not used that often, fortunately. If an Automated External Defibrillator is used, it is important that it does its job. That is why a defibrillator needs to be able to do self-testing on a regular basis.
- The Cardiac Science Powerheart G5 self tests daily itself for the following functionalities:
• Presence of electrodes.
• Functionality of the electrodes.
• The battery capacity.
• The capacitor frequency (monthly and weekly).
The Powerheart G5 defibrillator has an extremely accurate asystole detection with threshold measurement. This allows a shock to be applied to the weakest heart signal. This increases the chance of survival. In addition, this defibrillator can deliver a variable shock. This means that the power of each shock delivered is more powerful. Every body has a different resistance. Because the shock of the G5 is variable, this is handled in a smart way.
This makes this one of the most reliable AEDs available. Cardiac Science is the only AED manufacturer that gives a 4-year unconditional warranty on its batteries. If the Battery prompts for replacement before its 4th anniversary, you will receive a new battery with another 4-year guarantee perpetually.
Cardiac Science Powerheart G5 AED with CPR sensor
Special Features
- Delivers customized variable escalating energy from 95 to 354 Joules.
- Delivers Post-CPR shock typically within 10 seconds or less
- Rescue-Ready Indicator visibly indicates if the AED has successfully passed a self-test within past 24 hours.
- The G5 comes with a Medical-Grade battery with a 4-year Operating Guarantee and can deliver 420 shocks at 354 joules per shock
- The G5 prompts if it needs service, if the pads are tampered or disconnected, before the pads expire or if there is only 1 bar left on its battery gauge. At
1 bar, the G5 still has 9 shocks left at 354 joules per shock. It has built-in indicators which light up to show which part needs attention (AED, Pads or
Battery). This provides time to receive new pads or batteries. - The G5 is IP55 Rated – Dust and Water protected.
- The G5 comes with an 8-year AED manufacturer’s warranty.
- Have text instructions for noisy environments or for hearing impaired rescuers.
Please call (02) 7585-4765
Or contact us via email form below
Other AED Brands

Cardiac Science
Cardiac Science, 100+ years of existence and a well-known leader for AEDs and diagnostic cardiac monitoring devices, providing clinicians with effective tools to identify and treat heart disease, assess results and help improve public health.

ZOLL Medical
ZOLL Medical Corp, a company of Asahi Kasei is a well-known player in the resuscitation industry. For almost 40 years they develop medical devices and software solutions.
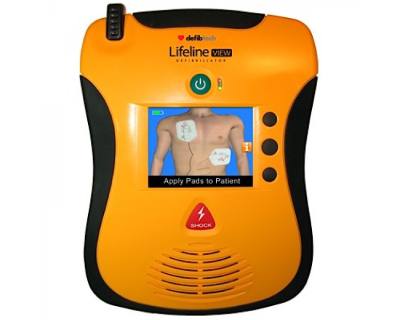
Defibtech
Defibtech is a leading innovator in the field of Automated External Defibrillators (AEDs). The company designs and manufactures the award-winning, authorized for marketing in the USA, Lifeline™ and ReviveR™ brands of defibrillators and related accessories.

Mindray
Mindray develops and manufactures medical devices and accessories for human and veterinary use. The company exists of three business lines: Patient Monitoring & Life Support, In-Vitro Diagnostic Products and Medical Imaging Systems.

Philips
Koninklijke Philips N.V. is a Dutch multinational conglomerate corporation, originated in Eindhoven, Netherlands. Philips is organized into two main divisions: Philips Consumer Health and Well-being and and Philips Professional Healthcare.

Radian
Radian Q Bio is established in 2005 in Korea. Their business activities are research, development, manufacturing of sensors, medical devices and measuring equipment. Since 2014 Radian included the Heart Guardian AED series to their product portfolio.
