 Defibtech Lifeline VIEW AED
Defibtech Lifeline VIEW AED
Defibtech Lifeline VIEW AED
- This AED package includes:
- Defibtech Lifeline VIEW semi-automatic AED
- Defibtech Lifeline VIEW battery
- Defibtech Lifeline VIEW elektrodes for Adults
- Defibtech Lifeline View Manual
Defibtech Lifeline VIEW AED
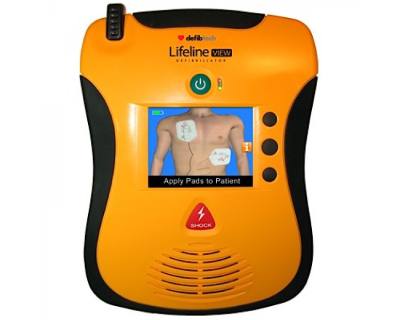
Defibtech Lifeline VIEW AED with Big LCD display screen
The Defibtech Lifeline View Semi-Automatic is a easy to use, yet advanced AED. Operation is very easy thanks to the colorful LCD display and spoken voice commands.
The Defibtech Lifeline View Semi-Automatic contains a red shock button. If the AED is connected to a victim with heart failure and recommends a shock, the rescuer should press the shock button. The AED will determine the best moment of shock and deliver it if necessary.
The Defibtech Lifeline View AED is designed from a user point of view. A deployment of an AED is a stressful situation. By designing the AED as user-friendly as possible, the care provider is guided as well as possible. The Defibtech Lifeline VIEW AED does this by guiding the rescuer with both voice commands and visual expressions that appear on the display.
The defibrillator is bilingual, though in the Philippines it’s offered with English Voice instructions only.
This Automated External Defibrillator is FDA certified. This means that the AED is produced according to the strictest requirements. In addition to certification, reliability is determined by self-testing. An AED is not used daily and therefore there is less insight into how the AED works when it is really needed. The presence of the electrodes and the capacity of the battery are tested automatically every day. Unfortunately, the defibrillator does not test for the functionality of the electrodes. The capacitor is, however, tested monthly by the self-tests.
The Defibtech Lifeline View AED Semi-Automatic has a high IP value. This means that the AED can be placed in both dusty areas and is well protected against rain.
The Lifeline View can stand crushes up to 450 kilograms.
Defibtech Lifeline VIEW AED
Special Features Defibtech Lifeline VIEW AED
- WAVEFORM: Biphasic Truncated Exponential (Impedance compensated)
- ENERGY: Adult: 150 Joules
Child/Infant: 50 Joules (Nominal into 50 Ohm load) - CHARGE TIME: Less than 4 seconds (shock decision to shock)
- RESCUE PROTOCOL: AHA/ERC 2010 or AHA 2015 guidelines (easily switchable during operation)
- CPR COACHING: Video and voice coaching On-demand video help
- VOICE PROMPTS: Extensive voice prompts guide user through operation of the unit
- VIDEO PROMPTS: Full motion video On-screen text prompts
- DISPLAY: High-resolution color LCD
- WARRANTY: 8 years on AED unit
Please call (02) 7585-4765
Or contact us via email form below
Other AED Brands

Radian
Radian Q Bio is established in 2005 in Korea. Their business activities are research, development, manufacturing of sensors, medical devices and measuring equipment. Since 2014 Radian included the Heart Guardian AED series to their product portfolio.
Physio Control
Physio-Control (Stryker), one of the leaders in the development, manufacturing, sale and service of external defibrillators, patient transport and data solutions. Well-known for their Heartsine AEDs and Lifepak defibrillators. Acquired by Stryker Corporation In 2016.

ZOLL Medical
ZOLL Medical Corp, a company of Asahi Kasei is a well-known player in the resuscitation industry. For almost 40 years they develop medical devices and software solutions.
Defibtech
Defibtech is a leading innovator in the field of Automated External Defibrillators (AEDs). The company designs and manufactures the award-winning, authorized for marketing in the USA, Lifeline™ and ReviveR™ brands of defibrillators and related accessories.

Mindray
Mindray develops and manufactures medical devices and accessories for human and veterinary use. The company exists of three business lines: Patient Monitoring & Life Support, In-Vitro Diagnostic Products and Medical Imaging Systems.

Cardiac Science
Cardiac Science, 100+ years of existence and a well-known leader for AEDs and diagnostic cardiac monitoring devices, providing clinicians with effective tools to identify and treat heart disease, assess results and help improve public health.
